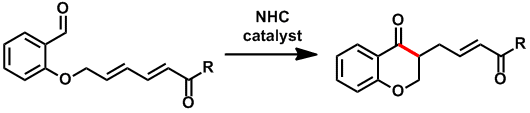
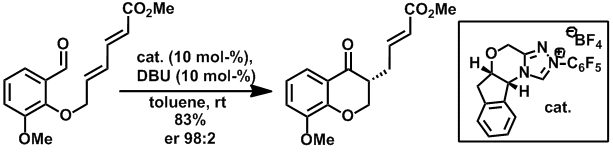
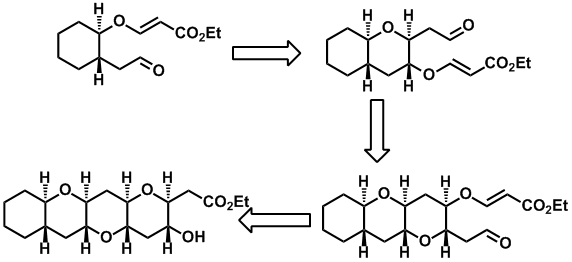
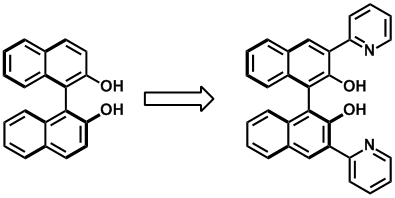
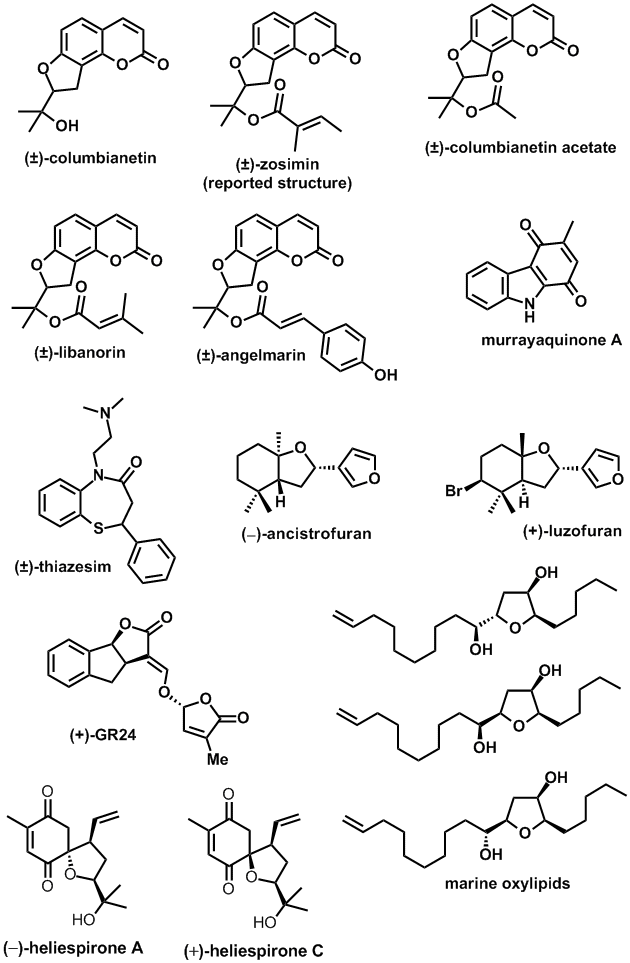
Synthesis of highly enantio-enriched stereoisomers of hydroxy-GR24. Joanne C. Morris and Christopher S. P. McErlean, Org. Biomol. Chem. (2016) 14, 1236–1238.
Expression of MAX2 under SCARECROW Promoter enhances the Strigolactone/MAX2 Dependent Response of Arabidopsis roots to Low-Phosphate Conditions. Ortal Madmon; Moram Mazuz; Puja Kumari; Anandamoy Dam; Aurel Ion; Einav Mayzlish-Gati; Eduard Belausov; Smadar Weininger; Mohamad Abu-Abied; Christopher McElrean; Liam Bromhead; Rafael Perl-Treves; Cristina Prandi; Yoram Kapulnik; Hinanit Koltai. Planta (2016) online 26/02/2016. DOI 10.1007/s00425-016-2477-7.
-
Chemistry of the synthetic strigolactone mimic GR24. Liam J. Bromhead, Jason Smith, and Christopher S. P. McErlean, Aus. J. Chem., (2015) 68, 1221-1227.[Invited article for Athel Beckwith Lectureship]
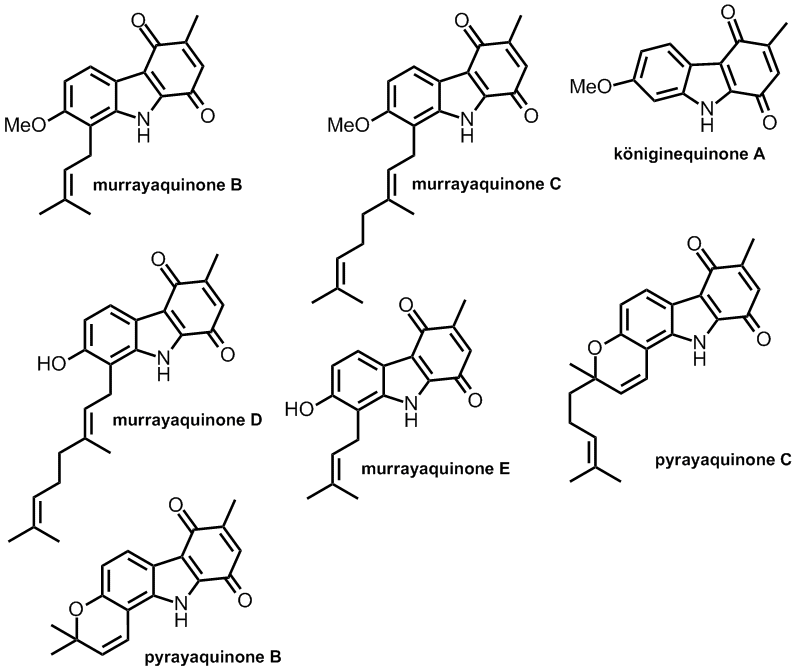
Synthesis of carbazoloquinone natural products ‘on-water’. Philip Norcott, Christopher S. P. McErlean. Org. Biomol. Chem., (2015) 13 , 6866-6878.
The response of Arabidopsis to low phosphate conditions that involves active changes in actin filaments and PIN2 polarization is dependent on strigolactone signaling. Manoj Kumar1, Nirali Pandya-Kumar1, Anandamoy Dam, Einav Mayzlish-Gati, Eduard Belausov, Smadar Wininger, Mohamad Abu-Abied, Liam J. Bromhead, Christopher S. P. McErlean, Cristina Prandi, Yoram Kapulnik and Hinanit Kolta. J. Exp. Bot., (2015) 66 , 1499-1510.
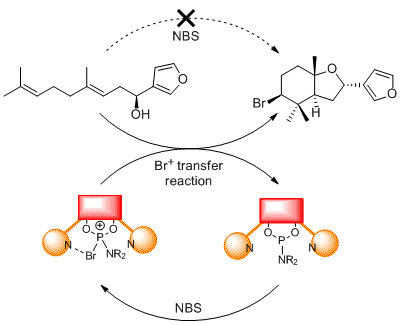
Accessing brominated natural product motifs using phosphoramidite-catalysis. Carl Recsei and Christopher S. P. McErlean, Aus. J. Chem., (2015) 68 555–565. [Invited article for Cornforth Memorial Issue]
Total Synthesis of (−)-Heliespirone A and (+)-Heliespirone C. Philip Norcott, Christopher S. P. McErlean Eur. J. Org. Chem., (2014), 5056–5062.
Avenaol, a germination stimulant for root parasitic plants from Avena strigos. Kim, H.I., Kisugi, T., Khetkam, P., Xie, X., Yoneyama, K., Uchida, K., Yokota, T., Nomura, T., McErlean, C.S.P., Yoneyama, K., Phytochemistry, (2014) 103, 85–88.
Synthesis of (+)-Luzofuran and (−)-Ancistrofuran. Carl Recsei, Bun Chan, and Christopher S. P. McErlean, J. Org. Chem., (2014) 79, 880–887.
Enantioselective synthesis of the strigolactone mimic (+)-GR24. Bromhead, Liam; Visser, Johan; McErlean, Christopher S. P., J. Org. Chem., (2014) 79, 1516–1520.
Dynamic imaging of the hepatitis C virus NS5A protein during a productive infection. Nicholas S. Eyre, Guillaume N. Fiches, Amanda L. Aloia, Karla J. Helbig, Erin M. McCartney, Christopher S. P. McErlean, Kui Li,
Anupriya Aggarwal, Stuart G. Turville, and Michael R. Beard, .
J. Virol., (2014) 88, 3636-3652.
Extending the Stetter Reaction with 1,6-Acceptors. Katherine R. Law, Christopher S. P. McErlean, Chem.-Eur. J. (2013) 19, 15852-15855.
Ionic liquids are compatible with on-water catalysis. Kaitlin D. Beare, Alexander K. L. Yuen, Anthony F. Masters, Thomas Maschmeyer and Christopher S. P. McErlean, Chem. Commun., (2013) 49, 8347-8349
A practical synthesis of (1S,4S)-2,5-diazabicyclo[2.2.1]heptane. Corinne Beinat, Samuel D. Banister, Christopher S. P. McErlean and Michael Kassiou, Tetrahedron Lett., (2013) 54, 5345-5347.
-
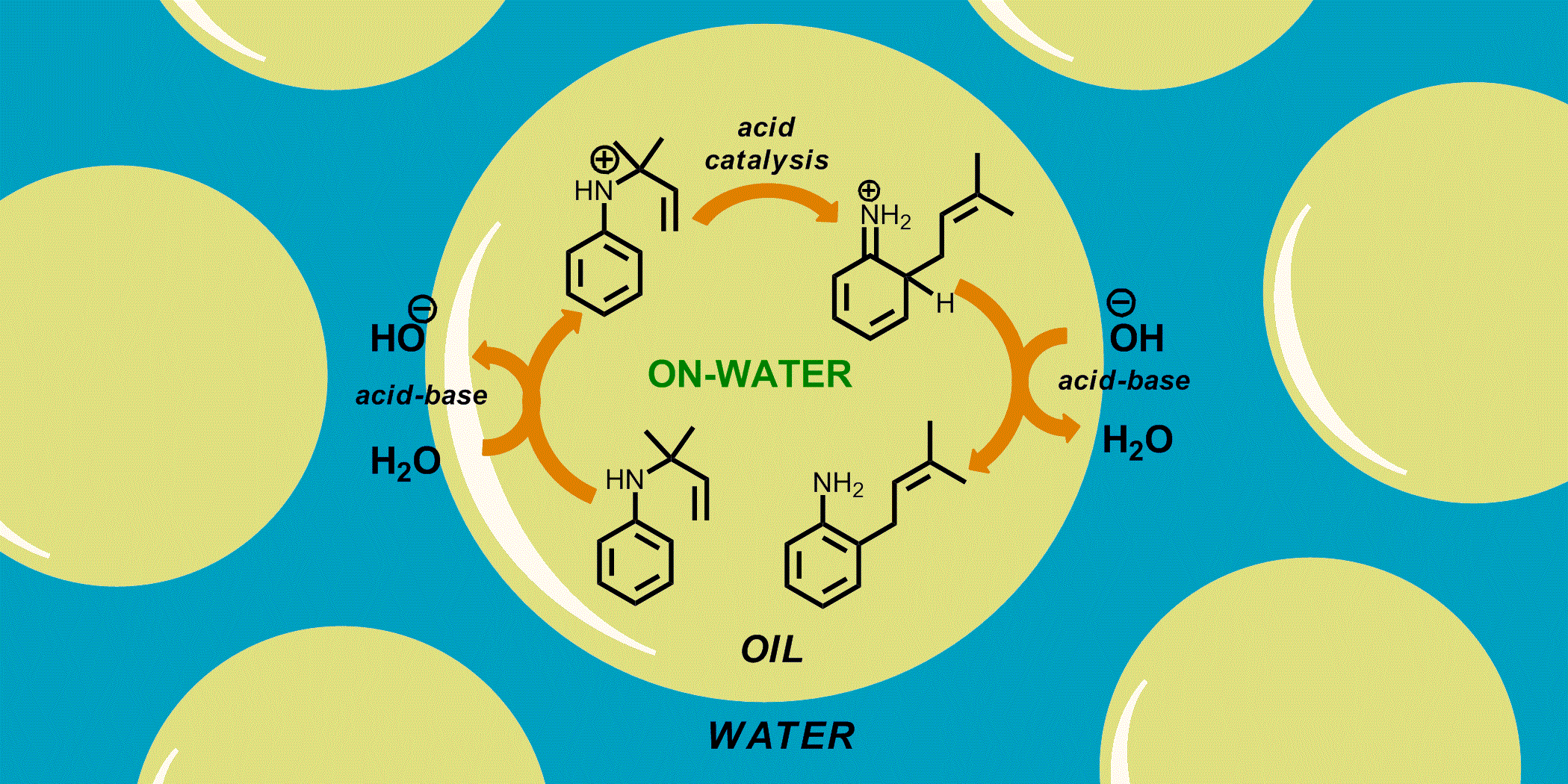
Revitalizing the aromatic aza-Claisen rearrangement: Implications for the mechanism of ‘on-water’ catalysis. Kaitlin D. Beare, Christopher S. P. McErlean, Org. Biomol. Chem., (2013) 11, 2452–2459.
-
Accessing Columbianetin-Containing Natural Products via a Domino On-water, In-Water Process. Kaitlin D. Beare, Christopher S. P. McErlean, Tetrahedron Lett., (2013) 54, 1056–1058.
-
An in-water, on-water domino process for synthesis. Philip Norcott, Calan Spielman and Christopher S. P. McErlean Green Chem., (2012) 14, 605–609.
-
N-Acylpyrroles: More than amides. Anna M. Goldys, Christopher S. P. McErlean, Eur. J. Org. Chem.(2012) 1877–1888.
-
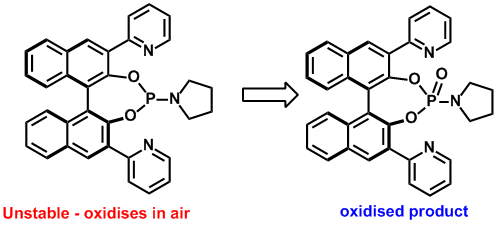
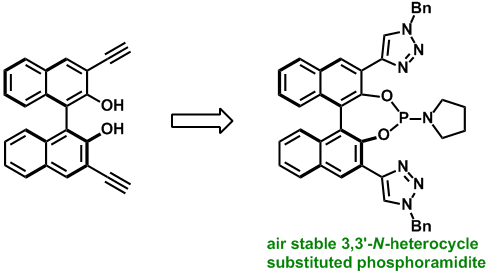
Synthesis of modified binol-phosphoramidites. Carl Recsei, Christopher S. P. McErlean, Tetrahedron (2012) 68, 464–480.
-
A Macrolactonization Approach to the Total Synthesis of LI-F04a and Diastereoisomeric Compounds. James R. Cochrane, Dong Hee Yoon, Christopher S. P. McErlean, Katrina A. Jolliffe. Beilstein J. Org. Chem. (2012) 8, 1344–1351.
-
Stetter Reactions of Unsaturated 1-Acyl-1H-pyrroles. Christopher B. W. Phippen, Anna M. Goldys, Christopher S. P. McErlean, Eur. J. Org. Chem. (2011) 6957–6964.
-
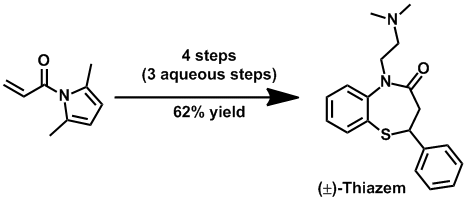
A 1,5-benzothiazepine synthesis. Christopher B. W. Phippen, Christopher S. P. McErlean,Tetrahedron Lett. (2011) 52, 1490–1492.
-
Total synthesis of C19 lipid diols containing a 2,5-disubstituted-3-oxygenated tetrahydrofuran. Caroline L Nesbitt, Christopher S. P. McErlean, Org. Biomol. Chem. (2011) 9, 2198–2208.
-
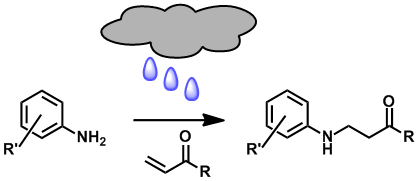
“On-Water” Conjugate Additions of Anilines. Christopher B. W. Phippen,James K. Beattie, and Christopher S. P. McErlean, Chem. Commun. (2010) 46, 8234–8236.
-
NR2B Selective Antagonists: Insights into Structure Activity Relationships and Therapeutic Applications. Corinne Beinat, Samuel Banister, Iman Moussa, Aaron J. Reynolds, Christopher S. P. McErlean, Michael Kassiou, Curr. Med. Chem. (2010) 17, 4166–4190.
-
Total Synthesis and Assignment of the Sidechain Stereochemistry of LI-F04a: An Antimicrobial Cyclic Depsipeptide. Cochrane, James R.; McErlean, Christopher S. P.; Jolliffe, Katrina A., Org. Lett. (2010)12, 3394–3397.
-
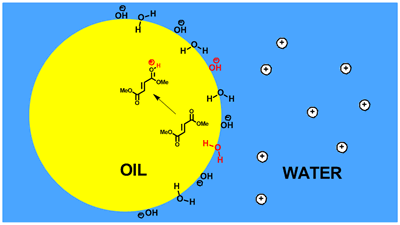
The mechanism of on-water catalysis. Beattie, James K.; McErlean, Christopher S. P.; Phippen, Christopher B. W., Chem.Eur. J. (2010) 16, 8972–8974.
-
Toward the synthesis of N-heterocycle containing BINOL-phosphoramidites. Recsei, Carl; McErlean, Christopher S. P., Tetrahedron: Asymmetry (2010) 21, 149–152.
-
An expedient synthesis of 2,5-disubstituted-3-oxygenated tetrahydrofurans. Nesbitt, Caroline L.; McErlean, Christopher S. P., Tetrahedron Lett. (2009) 50, 6318–6320.
-
An efficient synthesis of 3,3΄-dipyridyl BINOL ligands. Goldys, Anna; McErlean, Christopher S. P.,Tetrahedron Lett. (2009) 50, 3985–3987.
-
Application of an intramolecular Stetter reaction to access trans, syn, trans-fused pyrans. McErlean, Christopher S. P.; Willis, Anthony C., Synlett (2009) 233-236.
-
First synthesis of N-(3-carboxylpropyl)-5-amino-2-hydroxy-3-tridecyl-1,4-benzoquinone, an unusual quinone isolated from Embelia ribes. McErlean, Christopher S. P.; Moody, Christopher J., J. Org. Chem. (2007) 72, 10298–301.
-
Synthesis of the calothrixins, pentacyclic indolo[3,2-j]phenanthridine alkaloids, using a biomimetic approach. McErlean, Christopher, S. P.; Sperry, Jonathon; Blake, Alexander J.; Moody, Christopher J.Tetrahedron (2007) 63 (45), 10963–70.
-
Synthetic ansamycins prepared by a ring-expanding Claisen rearrangement. Synthesis and biological evaluation of ring and conformational analogues of the Hsp90 molecular chaperone inhibitor geldanamycin. McErlean, Christopher S. P.; Proisy, Nicolas; Davis, Christopher J.; Boland, Nicola A.; Sharp, Swee Y.; Boxall, Kathy; Slawin, Alexandra, M. Z.; Workman, Paul; Moody, Christopher J. Org. Biomol. Chem. (2007) 5 (3), 531–46.
-
A biomimetic synthesis of calothrixin B. Sperry, Jonathon; McErlean, Christopher, S. P.; Slawin, Alexandra, M. Z.; Moody, Christopher J. Tetrahedron. Lett. (2007) 48 (2), 231–34.
-
Rapid two-directional synthesis of the F-J fragment of the gambieric acids by iterative double ring-closing metathesis. Kimber, Marc C.; Robertson, Jerod; McErlean, Christopher, S. P.; Wilson, Claire; Clark, J. Stephen, Angew. Chem. Int. Ed. (2005) 44 (38), 6157-6162.
-
Spiroacetal Biosynthesis: (±)-1,7-Dioxaspiro[5.5]undecane in Bactrocera cacuminata and Bactrocera oleae (Olive Fruit Fly). Schwartz, Brett D.; McErlean, Christopher, S. P.; Flectcher, Mary T.; Mazomenos, Basilis E.; Konstantopoulou, Maria A.; Kitching, William; De Voss, James, J. Org. Lett. (2005) 7 (6), 1173-1176.
-
Insect chemistry and chirality. Hayes, Patricia Y.; Flectcher, Mary T.; Chow, Sharon; McGrath, Matthew J.; Tu, Yong, Q.; Zhang, Hesheng; Hungerford, Natasha L.; McErlean, Christopher, S. P.; Stok, Jeanette E.; Moore, Christopher J.; De Voss, James J.; Kitching, William, Chirality (2003) 15 suppl 1, S116–27.
-
Monooxygenase stereoselectivity in the biosynthesis of stereoisomeric spiroacetals in the cucmber fly, Bactrocera cucumis. McErlean CSP, Fletcher MT, Wood BJ, De Voss JJ, Kitching W, Org. Lett. (2002) 4 (16), 2775–78.
-
Sub-structure syntheses and relative stereochemistry in the bistramide (bistratene) series of marine metabolites. Gallagher PO, McErlean CSP, Jacobs MF, Watters DJ, Kitching W, Tetrahedron Lett. (2002) 43 (3), 531–35.
-
Synthesis and stereochemistry of some bicyclic gama-lactones from parasitic wasps (hymenoptera: Braconidae). Utility of hydrolytic kinetic resolution of epoxides and palladium(II)-catalysed hydroxycyclisation-carbonylation-lactonisatio of ene-diols. Paddon-Jones GC, McErlean CSP, Hayes P, Moore CJ, Konig WA, Kitching W, J. Org. Chem. (2001) 66 (22), 7487–95.
-
Synthesis and stereochemistry of insect derived spiroacetals with branched carbon skeletons. Tu YQ, Hubener A, Zhang HS, Moore CJ, Fletcher MT, Hayes P, Dettner K, Francke W, McErlean CSP, Kitching Synthesis (2000) 13, 1956-1978